Polyethylene Glycol Fda Warning
Polyethylene glycol fda warning. Tell your doctor if you are pregnant or plan to become pregnant while using this medication. Other scientists meanwhile are not convinced PEG is involved at all. Tell your doctor if you are breast-feeding a baby.
Polyethylene Glycol Electrolytes is a prescription medication and over-the-counter medication used to treat Constipation and for Bowel Preparation. Unfortunately despite all of the above issues and the FDAs published warning related to polyethylene glycol safety you still wont find a hint of concern in MirLAXs patient literature. Do not use polyethylene glycol 3350 more than once per day.
Occasionally Polyethylene Glycol 3350 USP Powder for Oral Solution may cause nausea stomach fullness cramping diarrhea andor gas. 23 PEGs are water-soluble polymers that can form hydrogen bonds in a ratio of 100 water molecules per one PEG molecule. It is not known whether polyethylene glycol 3350 will harm an unborn baby.
6 2015 -- The safety of an ingredient in an adult laxative commonly given to children is being investigated the US. 172820 Polyethylene glycol mean molecular weight 200-9500. Polyethylene glycol 3350 may also be used for purposes not listed in this medication guide.
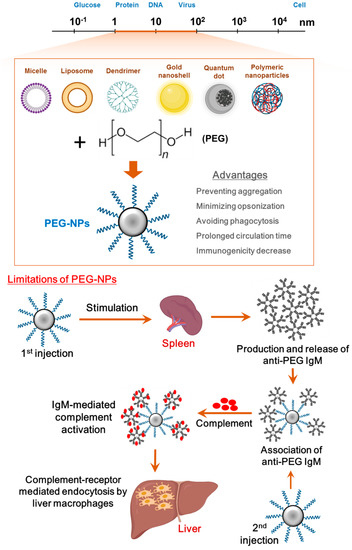
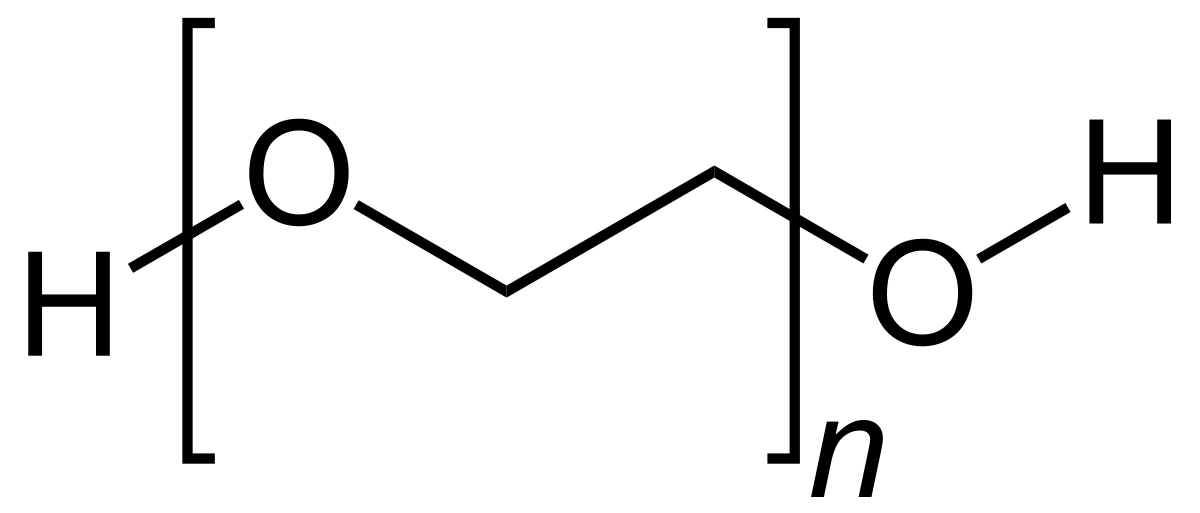
1 2 Several studies have confirmed its efficacy safety and superiority over other commonly used laxatives in children. Polyethylene glycol PEG is a synthetic polymer produced via polymerization of ethylene oxide molecules to make joining units of ethylene glycol by an ether linkage. If you have any of these conditions you could have dangerous or life-threatening side effects from polyethylene glycol 3350.
Food and Drug Administration says. Stir and dissolve the contents of one pouch 17 g into any 4 to 8 ounces of beverage cold hot or room temperature then drink 2. In no part of the document did we state that it was a class effect and made efforts to clearly indicate which UEAs contain PEG.
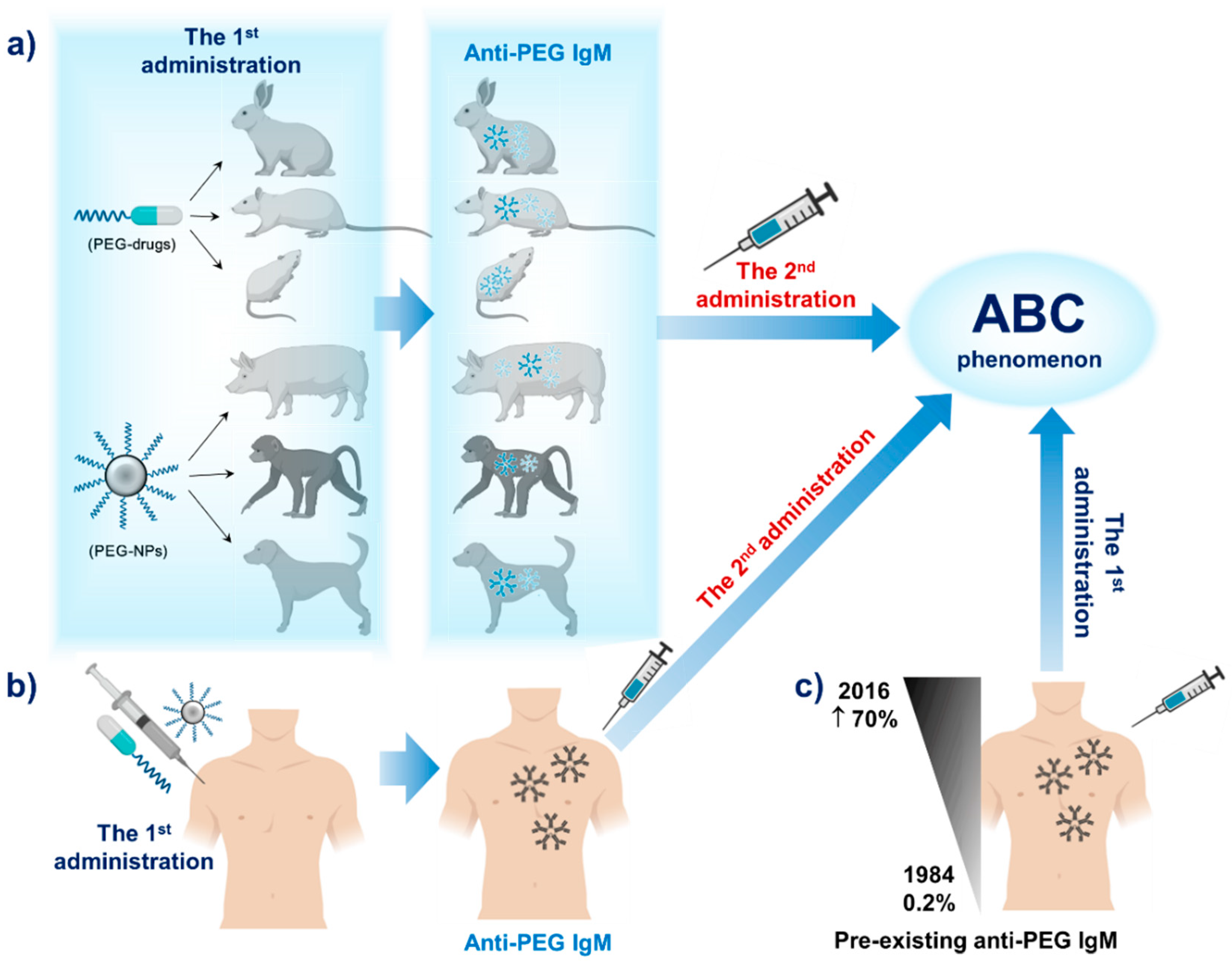
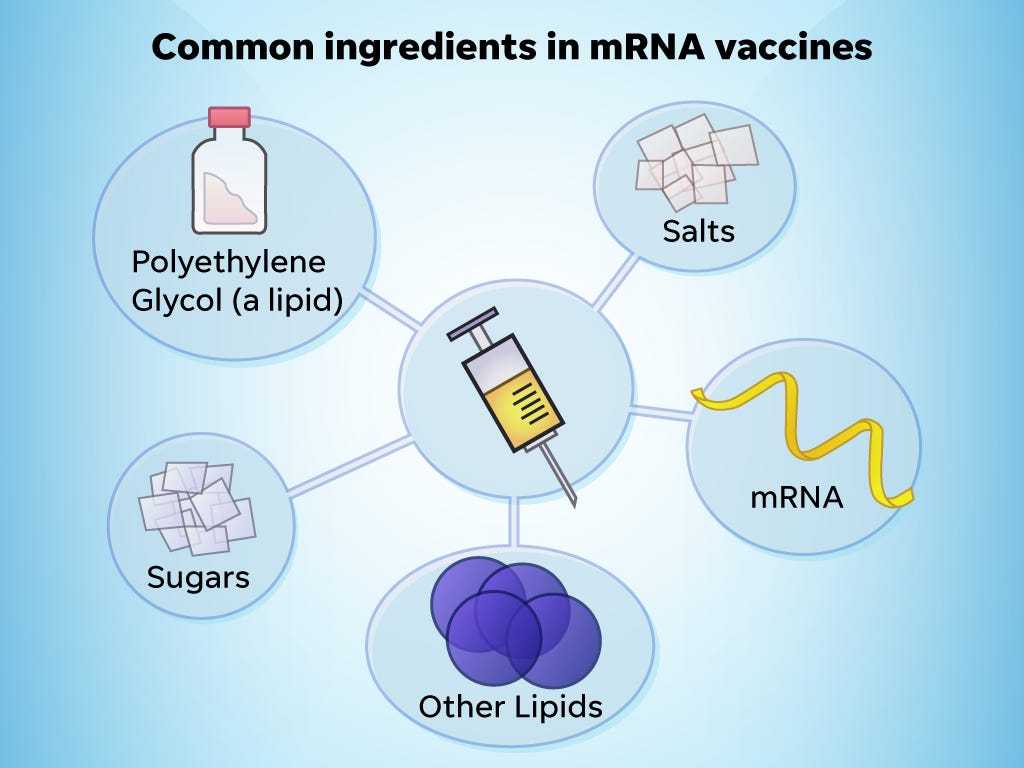
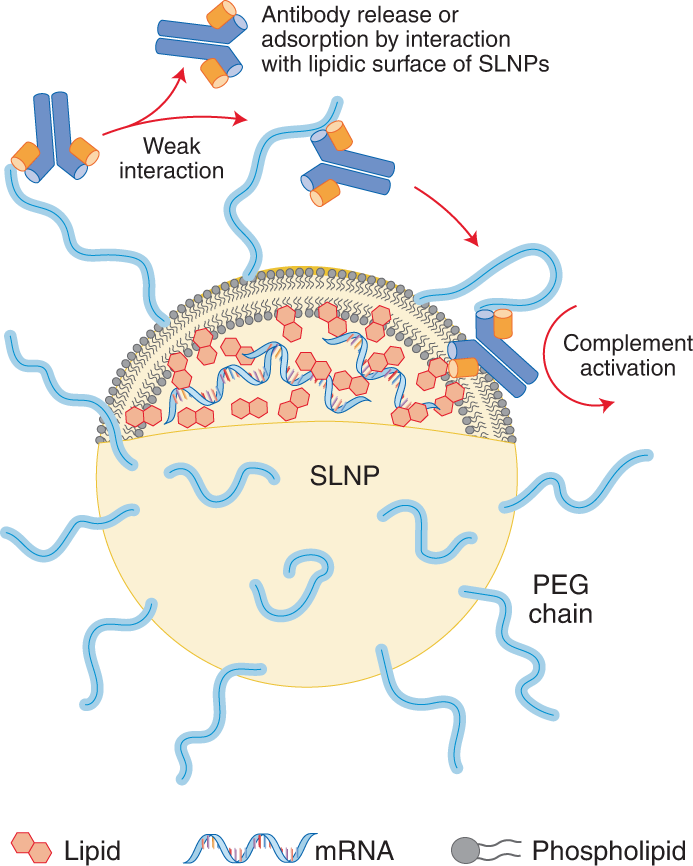
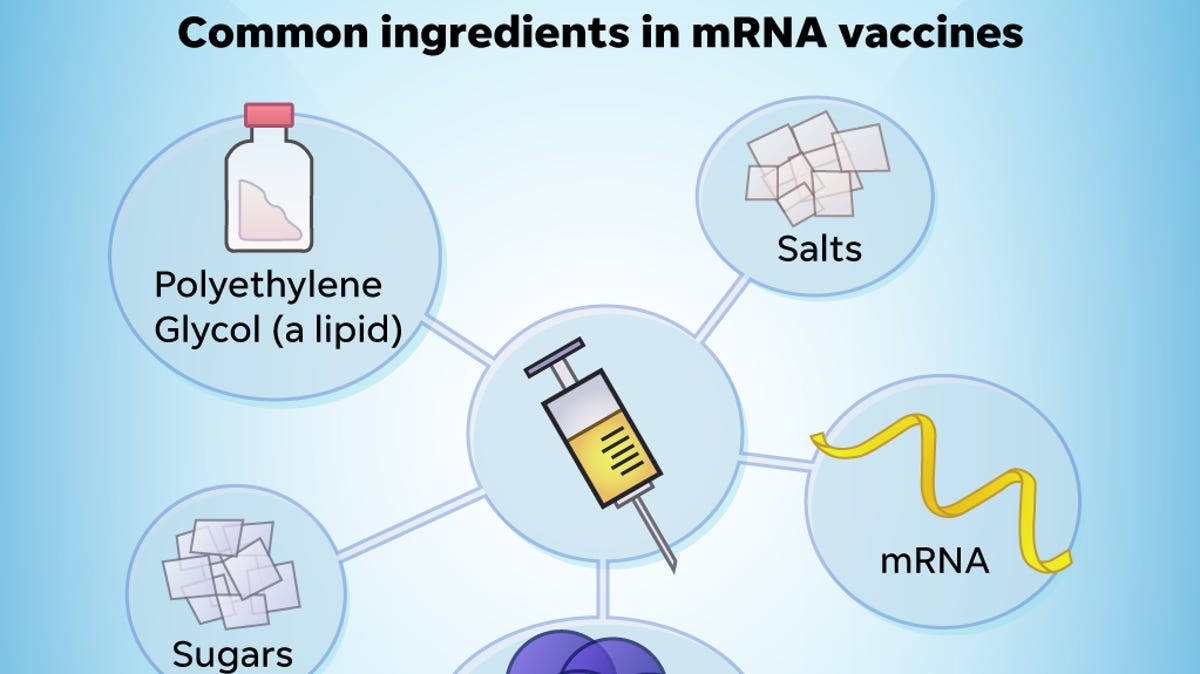
PEG is an ingredient in the mRNA vaccines and polysorbate is an ingredient in the JJJanssen vaccine. 2 Molecular weights of PEGs vary by time of the polymerization process and the molecular.
In no part of the document did we state that it was a class effect and made efforts to clearly indicate which UEAs contain PEG.
It is not known whether polyethylene glycol 3350 passes into breast milk or if it could harm a nursing baby. You should not use polyethylene glycol 3350 if you have a bowel obstruction or intestinal blockage. FDA pregnancy category C. 172820 Polyethylene glycol mean molecular weight 200-9500. Do not take if you have symptoms such as nausea vomiting abdominal pain or distention which may be due to bowel obstruction. Tell your doctor if you are breast-feeding a baby. 17 rows FDA is continuing to evaluate this issue to determine the need for any further. 9 gkg did not tolerate PEG 200 well and half of the animals had to be euthanized. If you have any of these conditions you could have dangerous or life-threatening side effects from polyethylene glycol 3350.
Polyethylene glycol 3350 PEG 3350. In no part of the document did we state that it was a class effect and made efforts to clearly indicate which UEAs contain PEG. MiraLAX is safe and effective and available without a prescription. In fact a Citizen Petition to Investigate Polyethylene Glycol 3350 Product Safety for Use with Pediatric Patients was filed back in 2012 urging the FDA to add a black box warning to its label pertaining to neuropsychiatric side effects in children. Propylene glycol is a compound which is GRAS generally recognized as safe by the US Food and Drug Administration under 21 CFR x1841666 and is also approved by the FDA for certain uses as an indirect food additive. 1 2 Several studies have confirmed its efficacy safety and superiority over other commonly used laxatives in children. Polyethylene glycol PEG is a synthetic polymer produced via polymerization of ethylene oxide molecules to make joining units of ethylene glycol by an ether linkage.











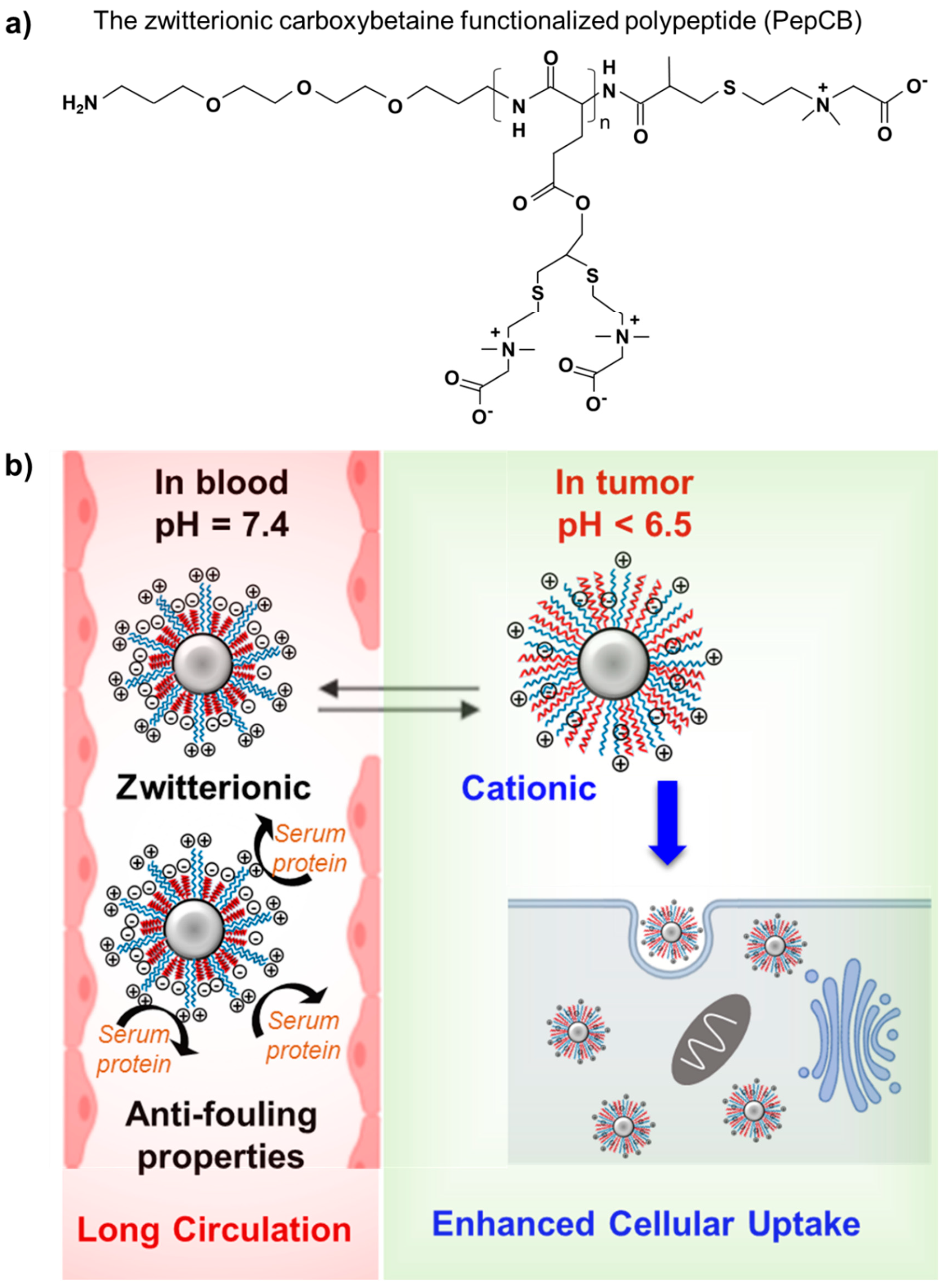



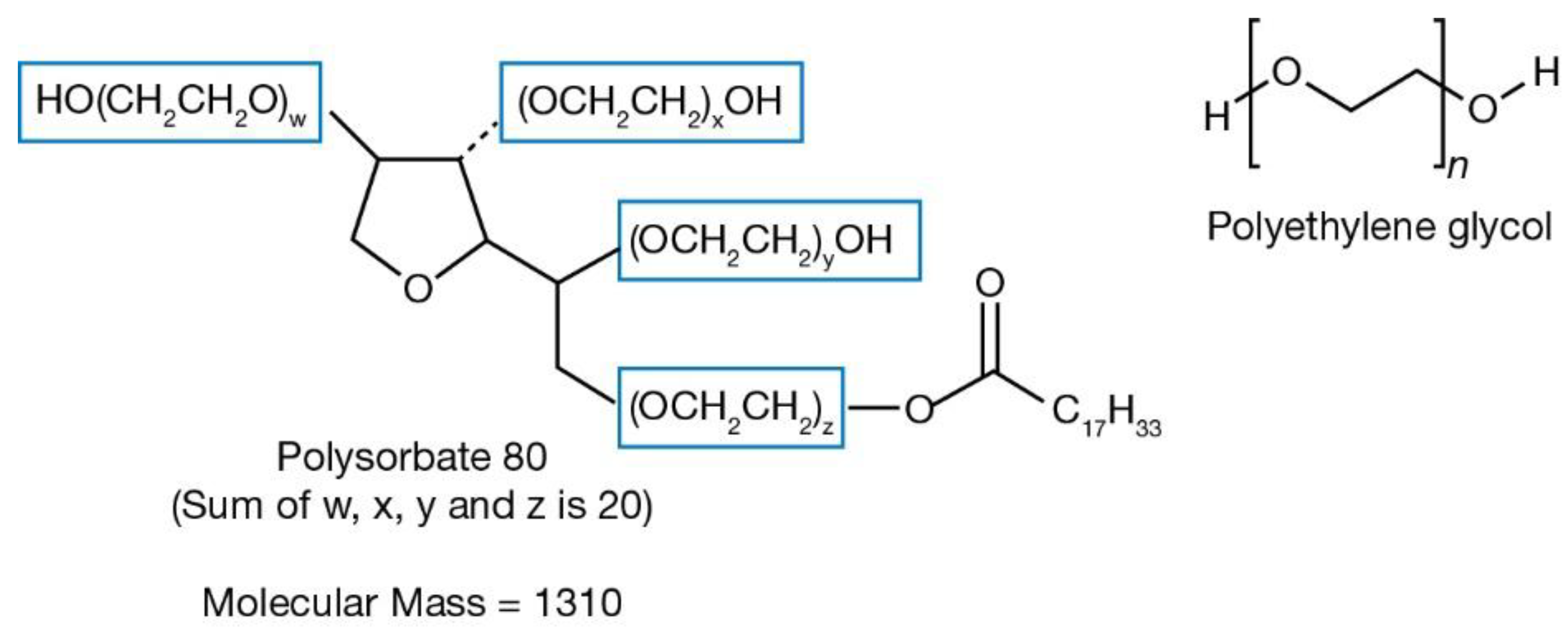




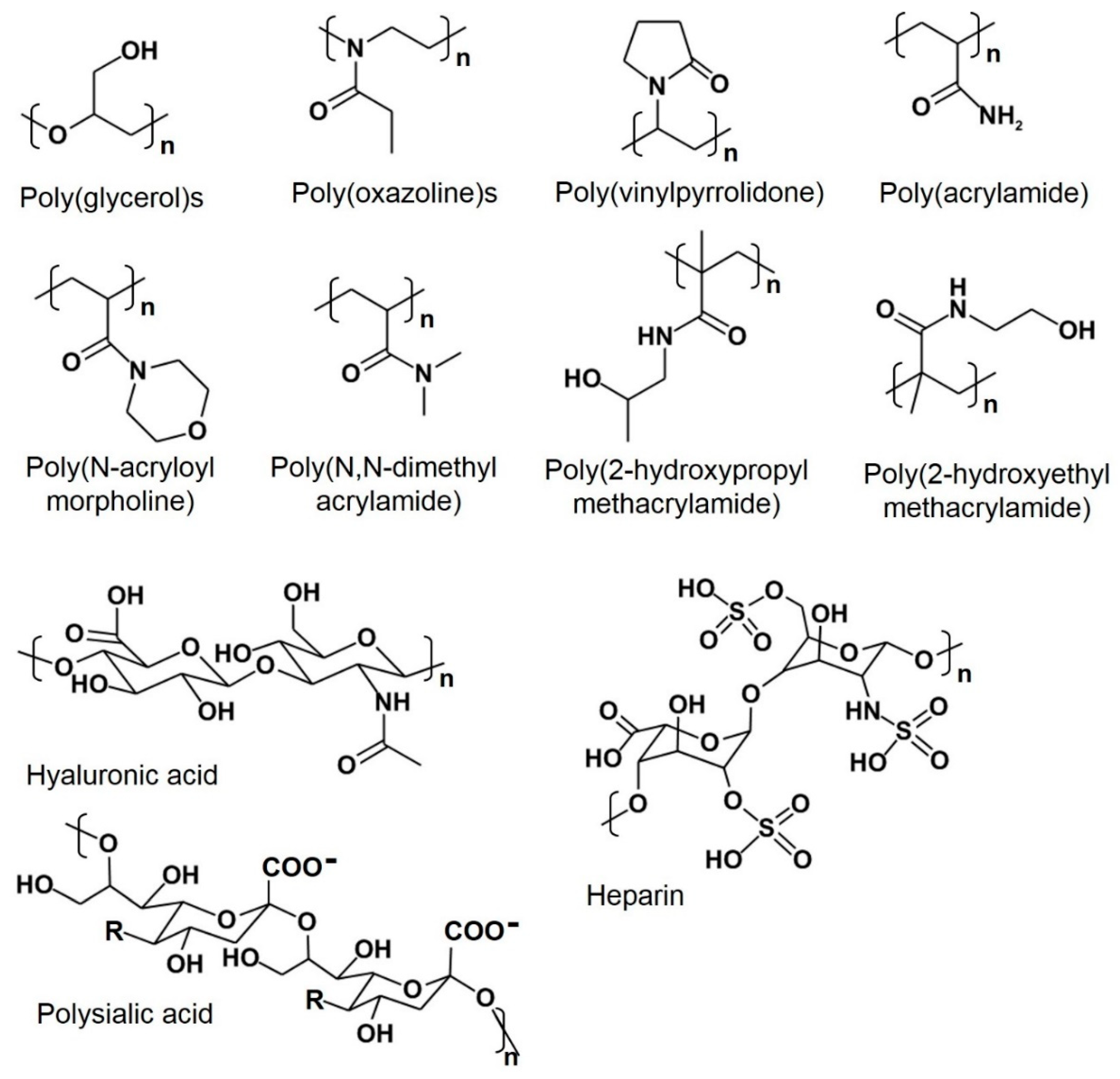
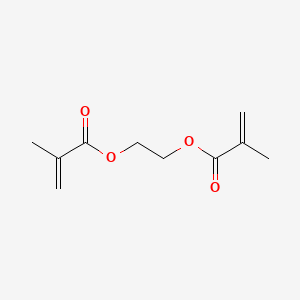









Post a Comment for "Polyethylene Glycol Fda Warning"